WHY TARA PV?
TARA PV was designed by a team of pharmacovigilance professionals who saw the benefits in a user-driven approach to processing and storing drug, device and vaccine adverse events in a secure safety database.
QUALITY ASSURED
TARA PV is a fully tested and audited pre-validated software system from an ISO 27001 accredited vendor.
FULLY COMPLIANT
TARA PV enables 21 CFR Part 11 compliance, adheres to GxP and ICH standards and allows compliance with all European & Worldwide regulations.
FAST AND INTEGRATED
As a hosted platform, TARA PV not only allows rapid implementation but also data migrations from other PV databases and third-party integration (eg Medidata RAVE).
FLEXIBLE LICENCE OPTIONS
TARA PV offers a range of pricing models, dependent on your requirements.
DATA ANALYSIS AND SIGNAL DETECTION
Microsoft Power BI is embedded in the TARA PV application providing a powerful data visualisation and interactive reporting tool.
COMPLETE CONFIGURATION
With an independent Administration Module and test database, TARA PV offers an integrated workflow with individual case assignment, company product dictionary and flexible configuration options. Also included as standard are the ISO E2B vocabulary lists.
CORE PV FUNCTIONALITY
Designed to meet international regulatory requirements including E2B(R3), CIOMS, MedWatch, PSUR and DSUR, with pre-submission case validation and action management and full MedDRA and WHODrug integration.
HOSTING PEACE OF MIND
Utilising ISO 27001 accredited Data Centres, we handle all routine server maintenance activities such as system critical security updates, triple layer backup, disaster recovery and GDPR/DPA compliance. Local hosting also available.
WHO IS TARA PV FOR?
Suitable for all sizes of pharmaceutical companies, CROs, academic / research institutions and other associated organisations.
Pharmaceutical Companies
Contract Research
Organisations
Academic & Research
Institutions
Including not for profit organisations

WHAT DO OUR CUSTOMERS SAY?
"TARA PV (Tools for Adverse Reaction Assessment) has been instrumental in delivering expected results through its quick speed and simplicity".
TAKE THE FIRST STEPOUR SPECIALITIES

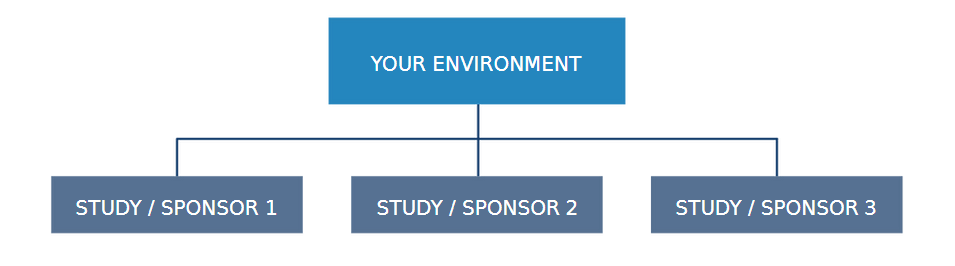
MULTITENANCY CAPABILITY
With our multitenancy functionality, you have the freedom to compartmentalise your entire PV system. By storing AER's within separate databases, maximum configurability is achieved, making this option ideal for defining allocated spaces for independent studies, sponsors or products.
We understand that everybody's needs are different and we therefore have pricing models to suit structures of all sizes. Please contact us for pricing options or request a demo with one of our specialists.

FLEXIBLE ADMINISTRATION
With an independent Administration Module, TARA PV offers extensive configuration and control over company data.
CONTACT US TO FIND OUT MOREDATA MANAGEMENT
As data analysis and signal detection capacity continues to be a priority for companies involved in pharmacovigilance, TARA PV continues to enhance our available data management tools.
With built in AI capabilities, our Power BI solution allows for customised report generation and simplified signal detection, ensuring streamlined process flows and the additional benefit of reduced human error. The creation and sharing of interactive visuals provides customised views of data giving you the flexibility of possessing complete control over your data management efforts. More importantly, with TARA PV, detailed analyses of adverse events is simplified without compromising the integrity of your data. Power BI is a powerful tool at your fingertips, included with all TARA PV installations.

INTUITIVE AI TECHNOLOGY
Automated signal detection and report generation for increased workflow efficiency and elimination of human error.
DOWNLOAD OUR DATASHEETDEVELOPMENT OF NEW FEATURES
With our UK based software development team, we are always enhancing TARA PV and have the capability to build whatever functionality you might require. We encourage you to contribute to the development roadmap, or alternatively, you are able to commission the development of your own enhancements.

ACTION MANAGEMENT
Stay organised by defining outstanding actions, tracking previous records and specifying user or group assignment, all in one user-friendly console.
SCHEDULE A DEMONSTRATIONLOCAL HOSTING

Local hosting is a option of increasing importance for some companies due to the dictation of certain legislations on data privacy and security by authorities across the globe. With TARA PV, our ability to host your environment on Microsoft Azure, fulfils these needs whilst meeting the applicable compliance guidelines.

ALL-INCLUSIVE TRAINING
Following implementation, we provide comprehensive training on our purposefully intuitive interface. We also offer refresher courses and supply additional resources such as video tutorials.
REQUEST A TAILORED COSTINGSSO FUNCTIONALITY
The integration of Single Sign-On is an optional feature that allows users to log into TARA PV using a single ID, associated with multiple independent systems within your organisation. Adding SSO authentication to your system provides an additional layer of security as well as streamlined processes and elevated productivity.

SUPPORT SPECIALISATION
Customer feedback highlights our ability to provide reliable, timely responses. When compared to other well-known organisations, our user support is unmatched.
DISCOVER MEDGENESISMIGRATE TO TARA PV
When working with new clients, we often encounter concerns surrounding the migration process. We understand the significance of a smooth transition from one system to another and after many years of experience, our tried and tested migration process is something on which you can rely.
We recognise that the onboarding process is critical, especially for those moving from an existing database that houses thousands of ICSR's. Utilising our experience in migrating cases from other systems, we are able to import data to ensure that your TARA PV implementation is as seamless as possible.
